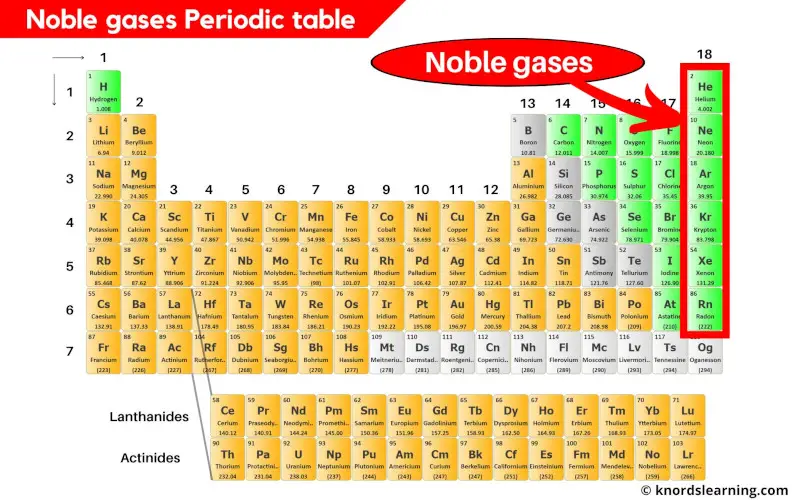
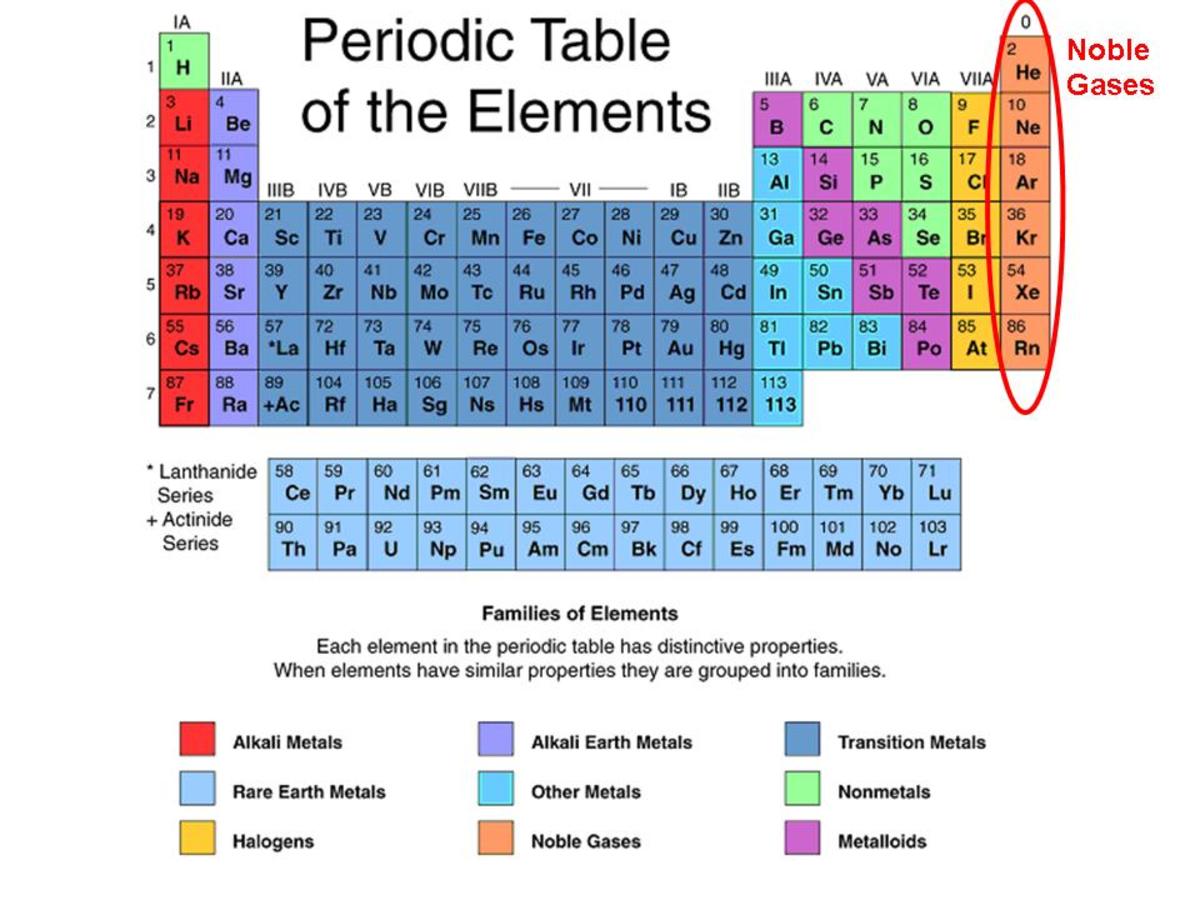
Do Noble Gases Form Ions - Helium, neon, argon, krypton and xenon are in. They show trends in their physical properties. The noble gases are in group 0 of the periodic table. They will not react with other atoms because they have a full outer shell of. Flame tests identify alkali metal ions in compounds. The noble gases are very unreactive.
They will not react with other atoms because they have a full outer shell of. The noble gases are in group 0 of the periodic table. The noble gases are very unreactive. They show trends in their physical properties. Helium, neon, argon, krypton and xenon are in. Flame tests identify alkali metal ions in compounds.
The noble gases are very unreactive. They show trends in their physical properties. The noble gases are in group 0 of the periodic table. Helium, neon, argon, krypton and xenon are in. They will not react with other atoms because they have a full outer shell of. Flame tests identify alkali metal ions in compounds.
Noble Gases Uses
The noble gases are in group 0 of the periodic table. The noble gases are very unreactive. Flame tests identify alkali metal ions in compounds. They show trends in their physical properties. Helium, neon, argon, krypton and xenon are in.
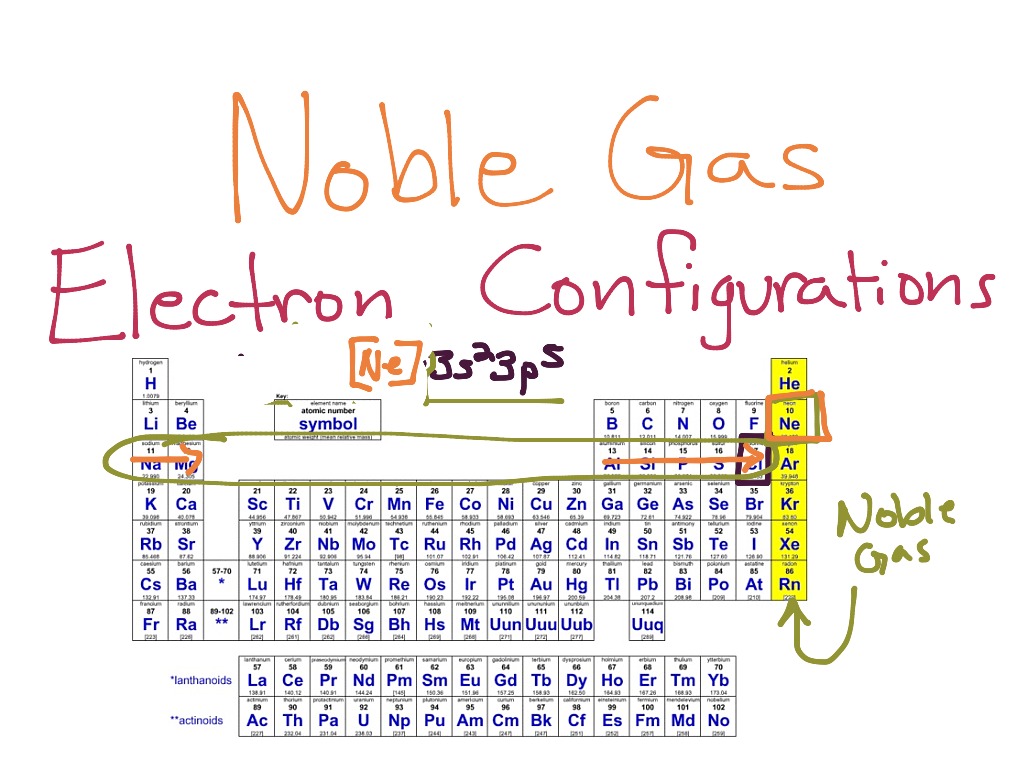
Noble Gases Electron Configuration
The noble gases are in group 0 of the periodic table. They show trends in their physical properties. Helium, neon, argon, krypton and xenon are in. The noble gases are very unreactive. They will not react with other atoms because they have a full outer shell of.
SOLVEDThe noble gases are sometimes called "inert gases." Why? Why do
The noble gases are in group 0 of the periodic table. Helium, neon, argon, krypton and xenon are in. Flame tests identify alkali metal ions in compounds. They show trends in their physical properties. They will not react with other atoms because they have a full outer shell of.
Noble Gases Periodic Table (With Images)
The noble gases are very unreactive. Flame tests identify alkali metal ions in compounds. They show trends in their physical properties. Helium, neon, argon, krypton and xenon are in. The noble gases are in group 0 of the periodic table.
Facts About the Noble Gases Learn Important Terms and Concepts
They show trends in their physical properties. The noble gases are very unreactive. Helium, neon, argon, krypton and xenon are in. The noble gases are in group 0 of the periodic table. They will not react with other atoms because they have a full outer shell of.
What's so Noble About Noble Gases? Owlcation
They will not react with other atoms because they have a full outer shell of. The noble gases are very unreactive. Flame tests identify alkali metal ions in compounds. Helium, neon, argon, krypton and xenon are in. The noble gases are in group 0 of the periodic table.
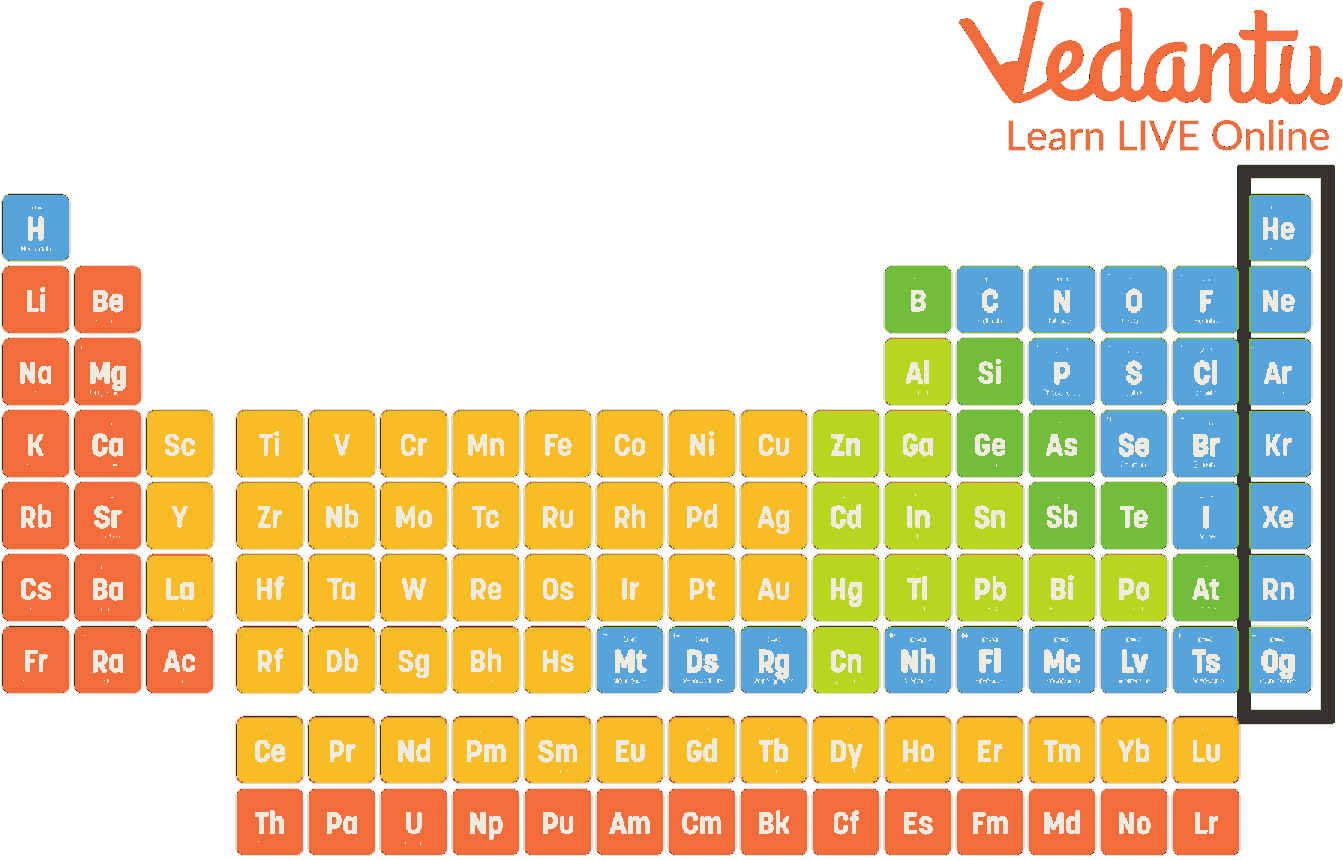
Periodic table Noble gases definition chemistry srshery
They will not react with other atoms because they have a full outer shell of. They show trends in their physical properties. Helium, neon, argon, krypton and xenon are in. The noble gases are very unreactive. Flame tests identify alkali metal ions in compounds.
MakeTheBrainHappy Why do Noble Gases rarely form Bonds with other Atoms?
They show trends in their physical properties. The noble gases are in group 0 of the periodic table. They will not react with other atoms because they have a full outer shell of. Flame tests identify alkali metal ions in compounds. Helium, neon, argon, krypton and xenon are in.
Noble Gas Chemical Compounds
They show trends in their physical properties. They will not react with other atoms because they have a full outer shell of. The noble gases are in group 0 of the periodic table. Flame tests identify alkali metal ions in compounds. The noble gases are very unreactive.
Noble Gases Properties, Applications, Effects
They show trends in their physical properties. The noble gases are in group 0 of the periodic table. The noble gases are very unreactive. They will not react with other atoms because they have a full outer shell of. Flame tests identify alkali metal ions in compounds.
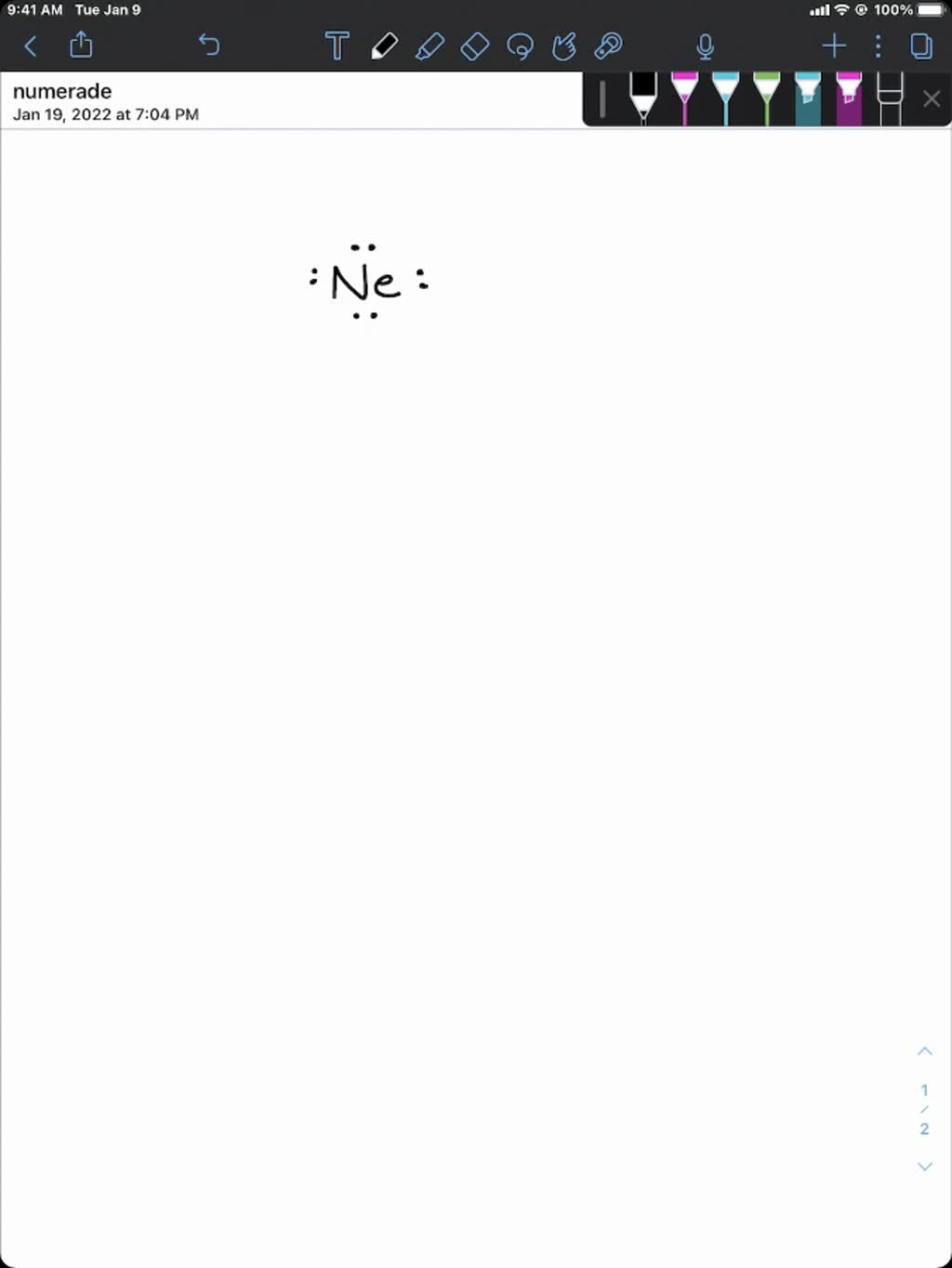
Helium, Neon, Argon, Krypton And Xenon Are In.
They show trends in their physical properties. The noble gases are very unreactive. The noble gases are in group 0 of the periodic table. They will not react with other atoms because they have a full outer shell of.


:max_bytes(150000):strip_icc()/Xenonhexafluoride-56a12d265f9b58b7d0bccc78.png)
