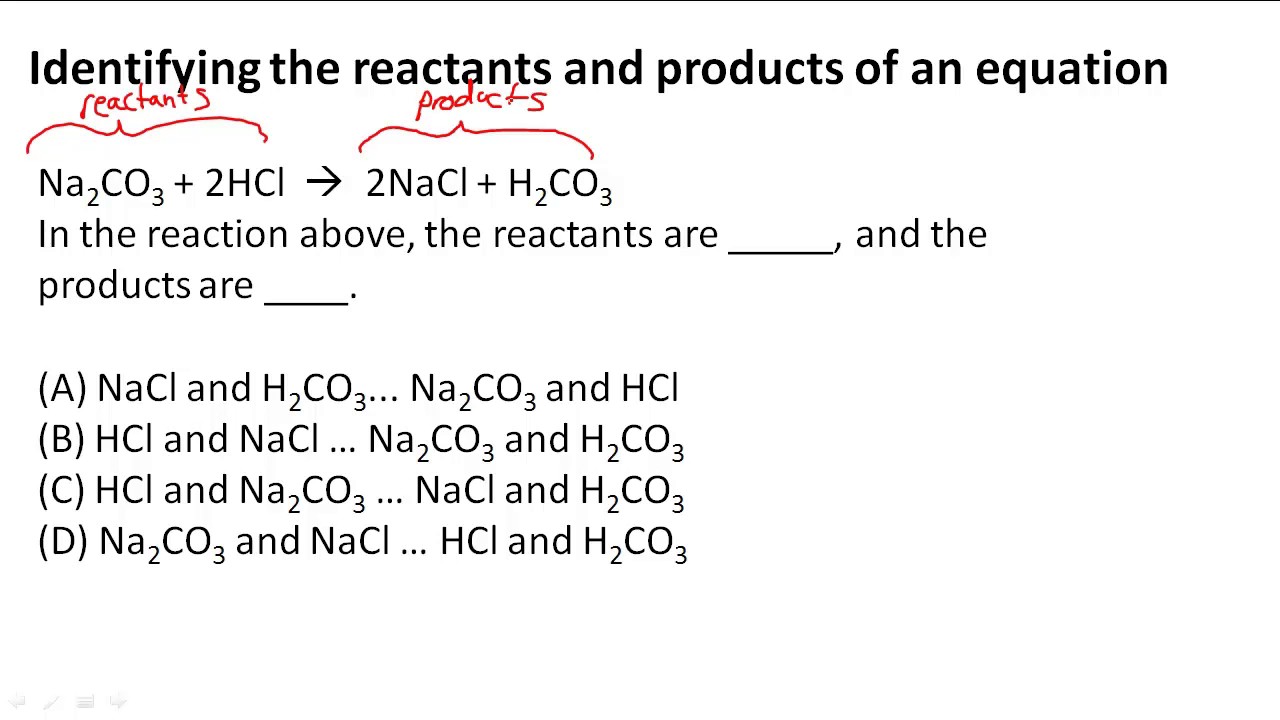
Two Or More Reactants Combine To Form One Product - When two or more simple reactants combine to form a new, more complex product, the reaction. What is a synthesis reaction? The type of chemical reaction in which two reactants combine to form one new. A process in which two or more chemicals combine to create a single new. A synthesis reaction is a reaction in which two or more. A synthesis reaction occurs when two or more reactants combine to form a single.
A synthesis reaction occurs when two or more reactants combine to form a single. When two or more simple reactants combine to form a new, more complex product, the reaction. A process in which two or more chemicals combine to create a single new. The type of chemical reaction in which two reactants combine to form one new. A synthesis reaction is a reaction in which two or more. What is a synthesis reaction?
A synthesis reaction is a reaction in which two or more. The type of chemical reaction in which two reactants combine to form one new. A process in which two or more chemicals combine to create a single new. When two or more simple reactants combine to form a new, more complex product, the reaction. A synthesis reaction occurs when two or more reactants combine to form a single. What is a synthesis reaction?
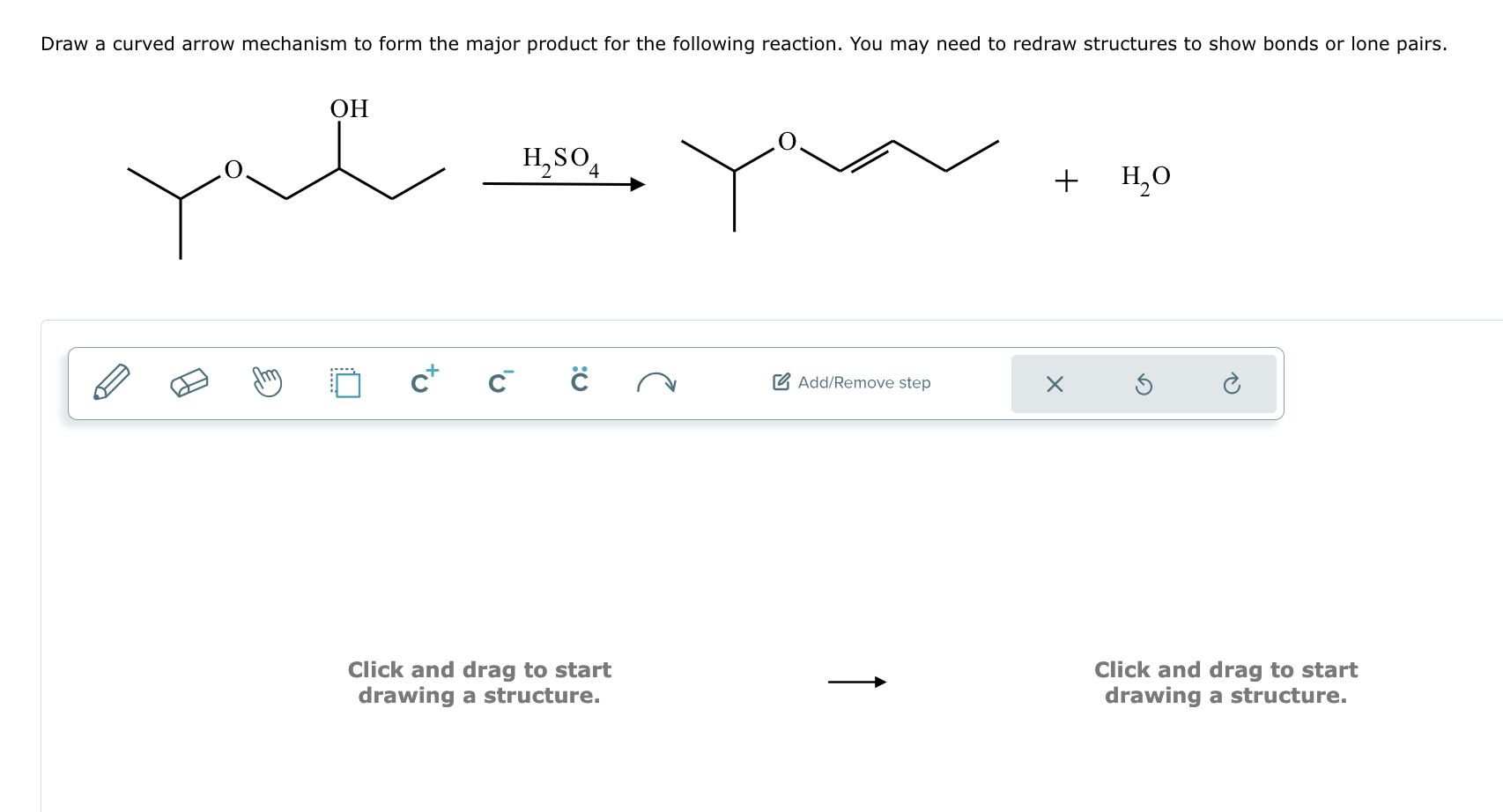
Solved Determine the reactants that produce the following products and
When two or more simple reactants combine to form a new, more complex product, the reaction. The type of chemical reaction in which two reactants combine to form one new. A process in which two or more chemicals combine to create a single new. What is a synthesis reaction? A synthesis reaction occurs when two or more reactants combine to.
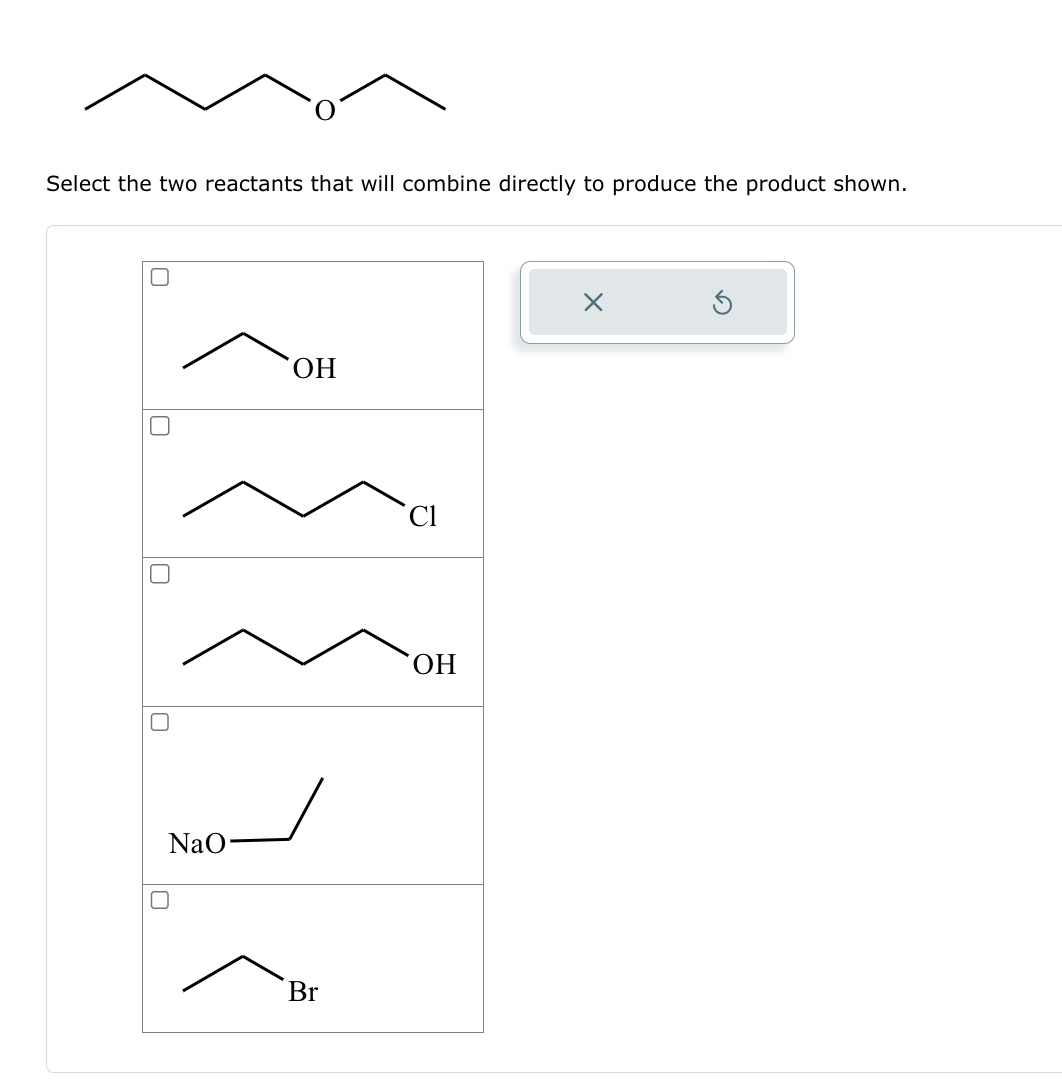
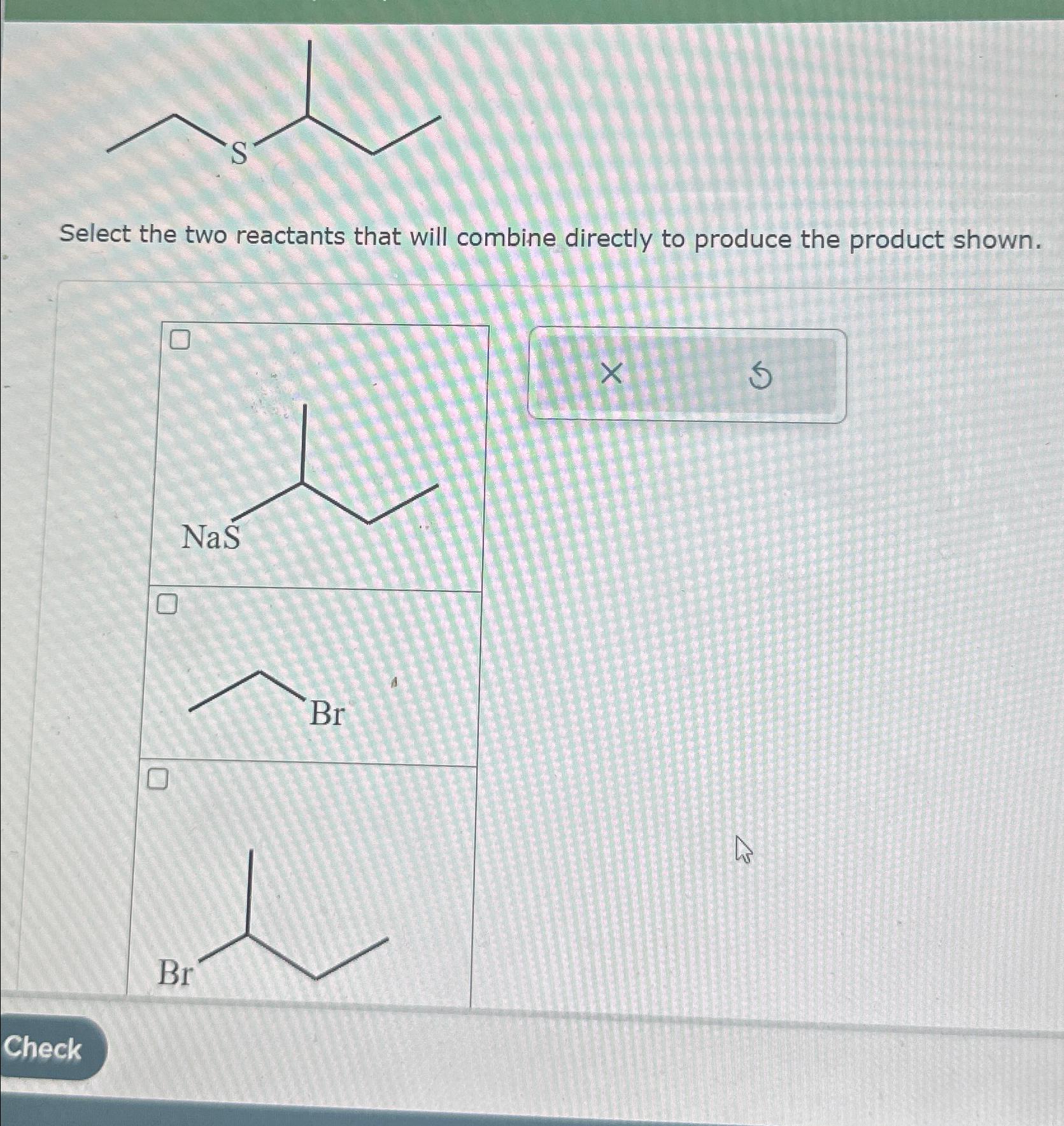
Solved Select the two reactants that will combine directly
The type of chemical reaction in which two reactants combine to form one new. When two or more simple reactants combine to form a new, more complex product, the reaction. What is a synthesis reaction? A synthesis reaction occurs when two or more reactants combine to form a single. A process in which two or more chemicals combine to create.
Synthesis Reactions occur when two of more reactants combine to
A synthesis reaction occurs when two or more reactants combine to form a single. What is a synthesis reaction? The type of chemical reaction in which two reactants combine to form one new. When two or more simple reactants combine to form a new, more complex product, the reaction. A synthesis reaction is a reaction in which two or more.
Reactants & Products of a Chemical Reaction Process & Examples
A process in which two or more chemicals combine to create a single new. The type of chemical reaction in which two reactants combine to form one new. A synthesis reaction occurs when two or more reactants combine to form a single. When two or more simple reactants combine to form a new, more complex product, the reaction. What is.
SOLVED Question 8 (5 points) Two reactants combine to form a product
When two or more simple reactants combine to form a new, more complex product, the reaction. What is a synthesis reaction? A synthesis reaction occurs when two or more reactants combine to form a single. A synthesis reaction is a reaction in which two or more. The type of chemical reaction in which two reactants combine to form one new.
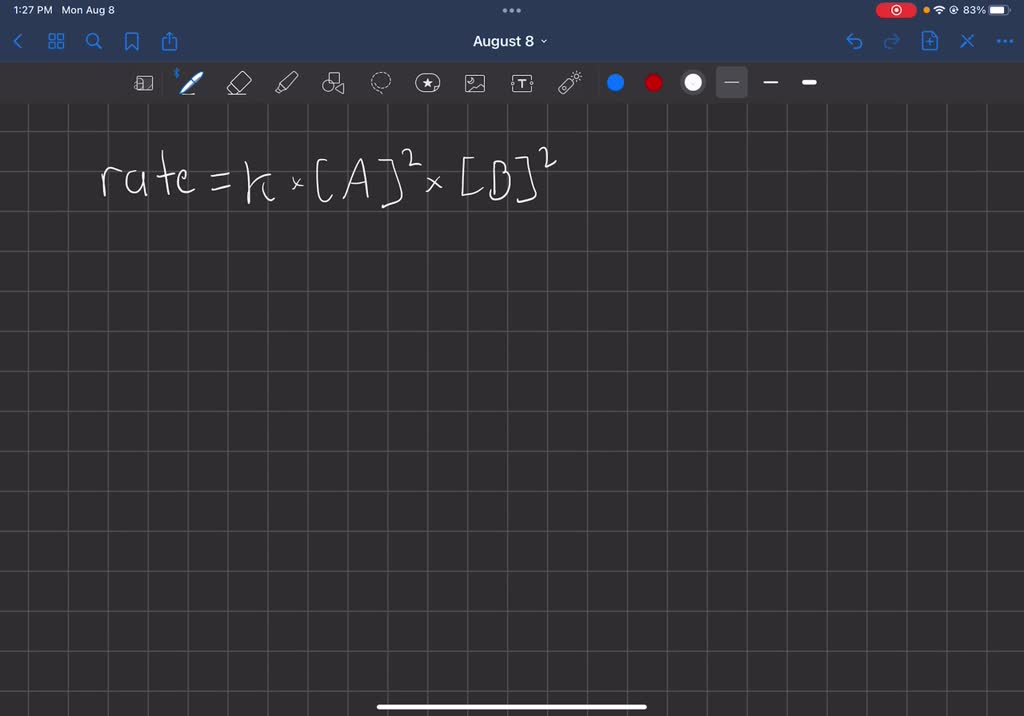
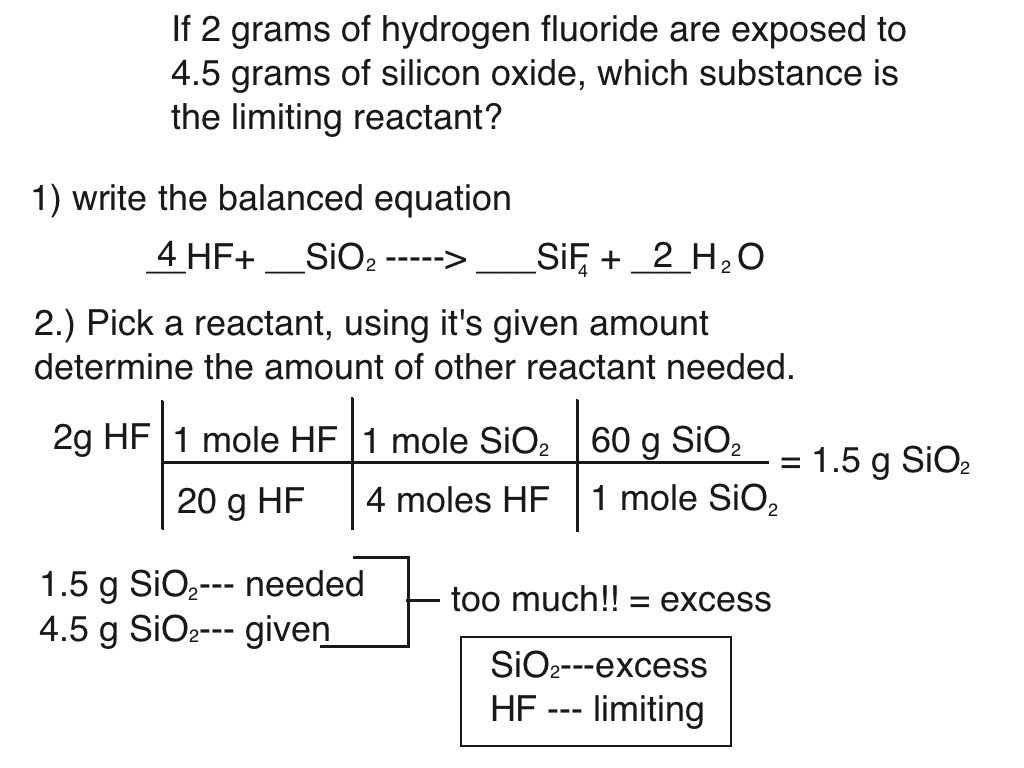
Limiting Reactants Chemistry 101
A synthesis reaction occurs when two or more reactants combine to form a single. A process in which two or more chemicals combine to create a single new. The type of chemical reaction in which two reactants combine to form one new. What is a synthesis reaction? A synthesis reaction is a reaction in which two or more.
Solved Select the two reactants that will combine directly
A synthesis reaction occurs when two or more reactants combine to form a single. A synthesis reaction is a reaction in which two or more. When two or more simple reactants combine to form a new, more complex product, the reaction. A process in which two or more chemicals combine to create a single new. What is a synthesis reaction?
What Is The Chemical Equation For Photosynthesis Identify Reactants And
What is a synthesis reaction? A synthesis reaction is a reaction in which two or more. The type of chemical reaction in which two reactants combine to form one new. When two or more simple reactants combine to form a new, more complex product, the reaction. A process in which two or more chemicals combine to create a single new.
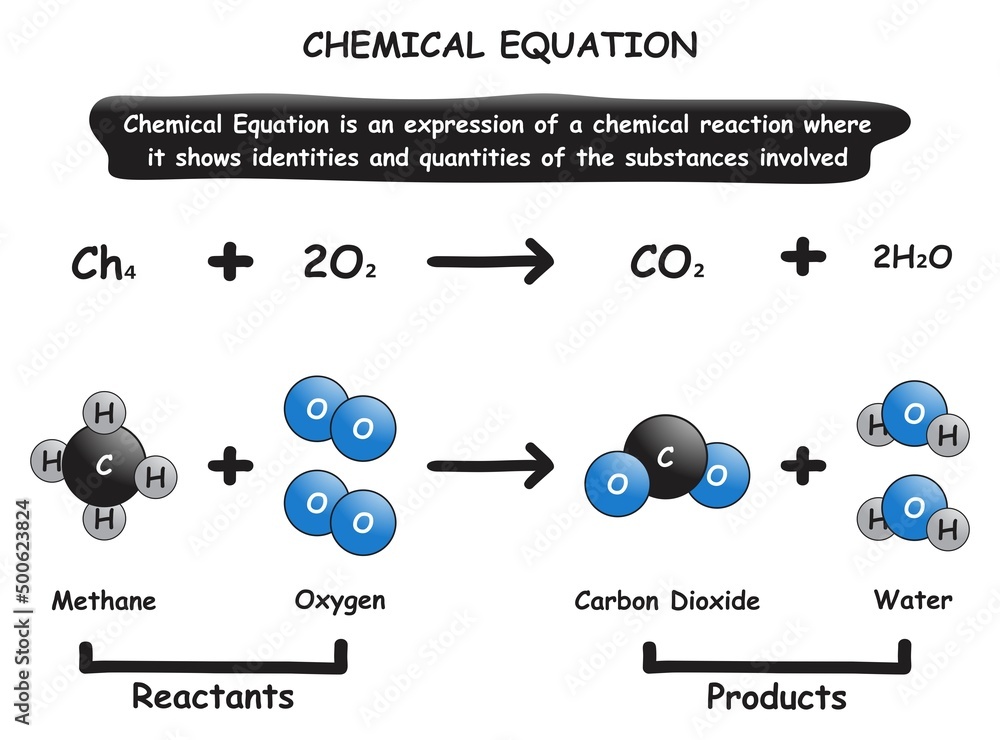
Chemical Equation Infographic diagram showing identities and quantities
A synthesis reaction is a reaction in which two or more. What is a synthesis reaction? The type of chemical reaction in which two reactants combine to form one new. A process in which two or more chemicals combine to create a single new. A synthesis reaction occurs when two or more reactants combine to form a single.
Solved Select the two reactants that will combine directly
The type of chemical reaction in which two reactants combine to form one new. What is a synthesis reaction? When two or more simple reactants combine to form a new, more complex product, the reaction. A process in which two or more chemicals combine to create a single new. A synthesis reaction occurs when two or more reactants combine to.
The Type Of Chemical Reaction In Which Two Reactants Combine To Form One New.
A process in which two or more chemicals combine to create a single new. When two or more simple reactants combine to form a new, more complex product, the reaction. A synthesis reaction occurs when two or more reactants combine to form a single. What is a synthesis reaction?