What Is Deprotection In Chemistry - When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones. Stability data for the most frequently used protective groups, protection and deprotection. Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. A protecting group or protective group is introduced into a molecule by chemical. Protecting groups are needed to temporarily block a certain reactive site on a molecule. Deprotection is the process of removing a protective group from a molecule, typically an.
A protecting group or protective group is introduced into a molecule by chemical. Stability data for the most frequently used protective groups, protection and deprotection. Protecting groups are needed to temporarily block a certain reactive site on a molecule. When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones. Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. Deprotection is the process of removing a protective group from a molecule, typically an.
Protecting groups are needed to temporarily block a certain reactive site on a molecule. Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. Stability data for the most frequently used protective groups, protection and deprotection. When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones. Deprotection is the process of removing a protective group from a molecule, typically an. A protecting group or protective group is introduced into a molecule by chemical.
RIP their deprotection r/chemistry
Stability data for the most frequently used protective groups, protection and deprotection. Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. Deprotection is the process of removing a protective group from a molecule, typically an. A protecting group or protective group is introduced into a molecule by chemical. When the alcohol is protected with a t.
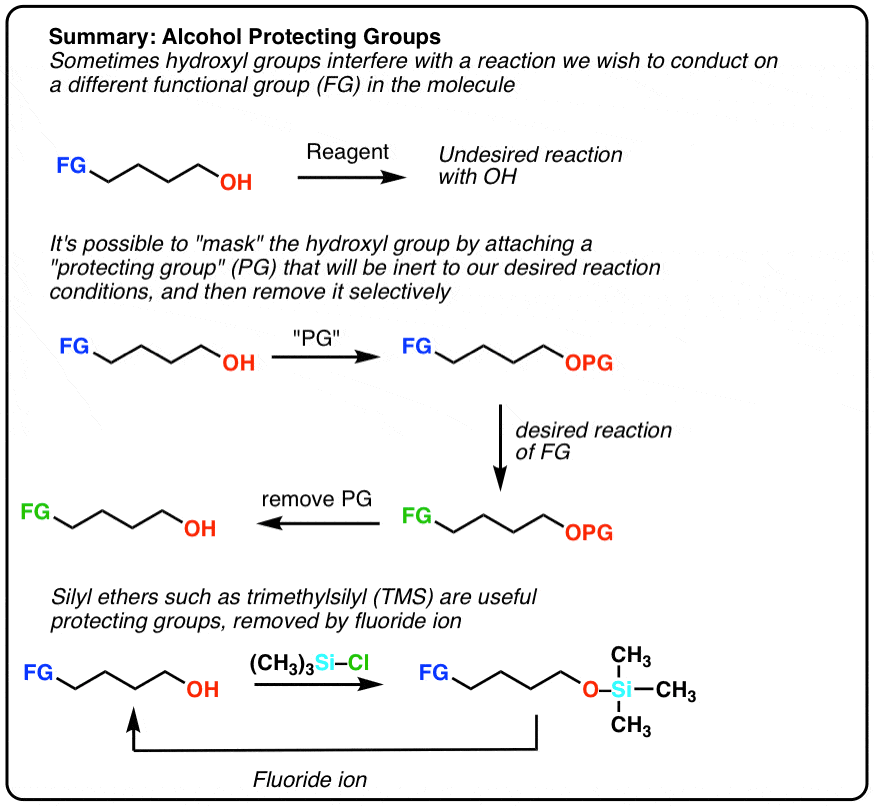
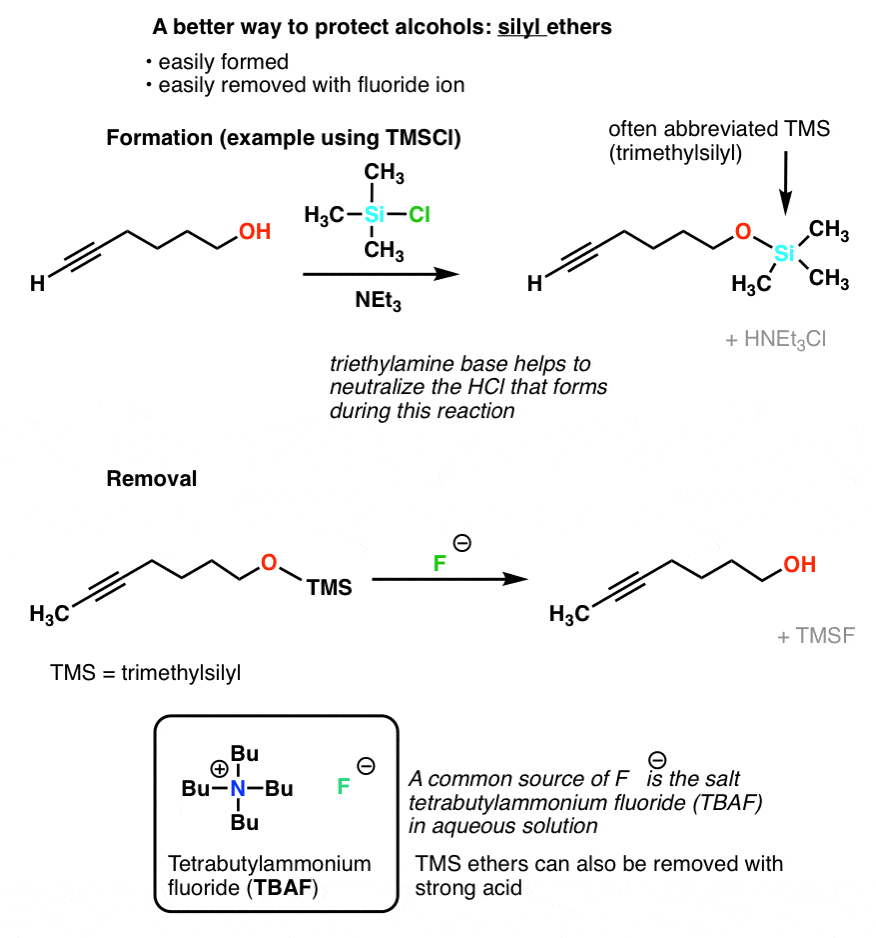
Protecting Groups For Alcohols Master Organic Chemistry
Protecting groups are needed to temporarily block a certain reactive site on a molecule. Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. Stability data for the most frequently used protective groups, protection and deprotection. When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones. A protecting group or.
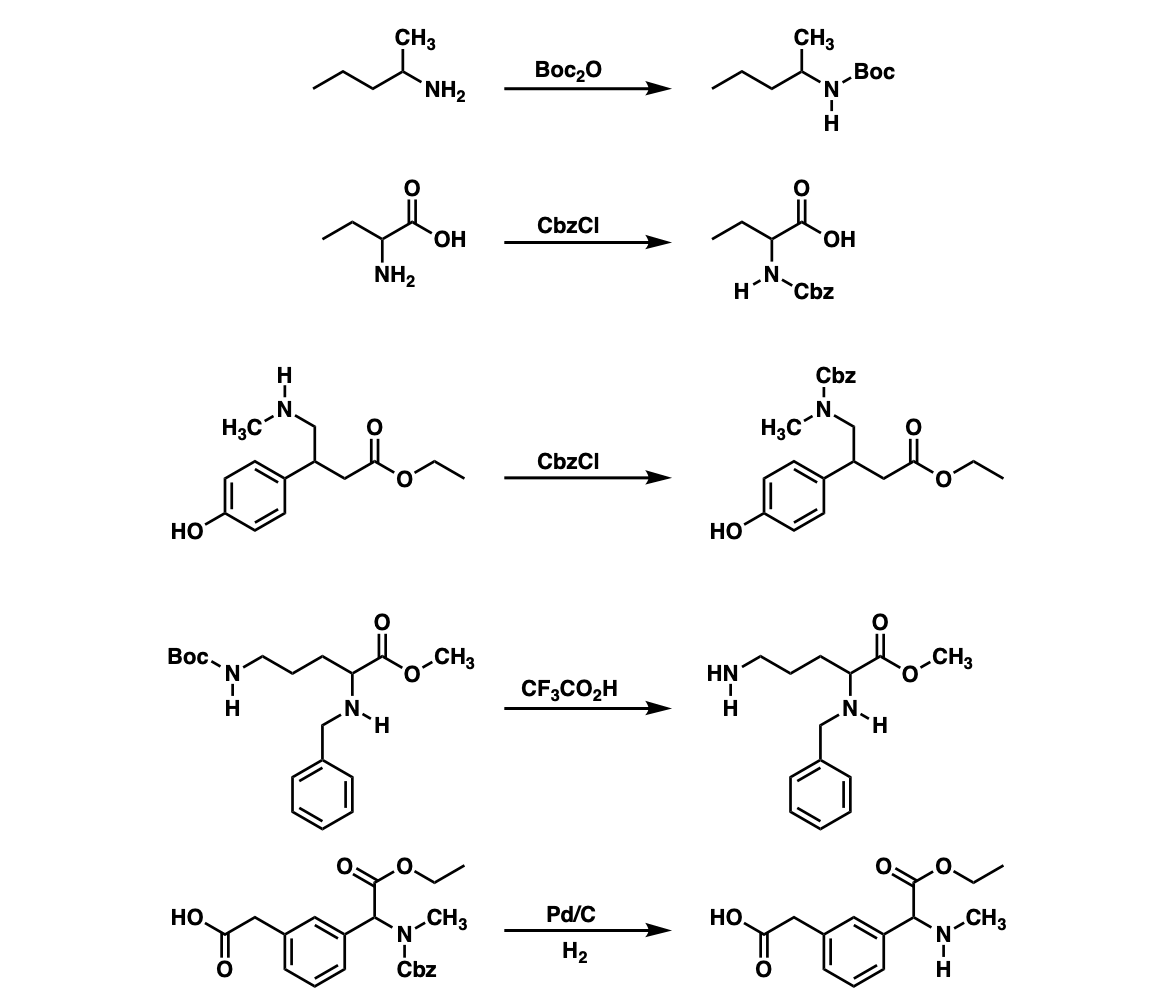
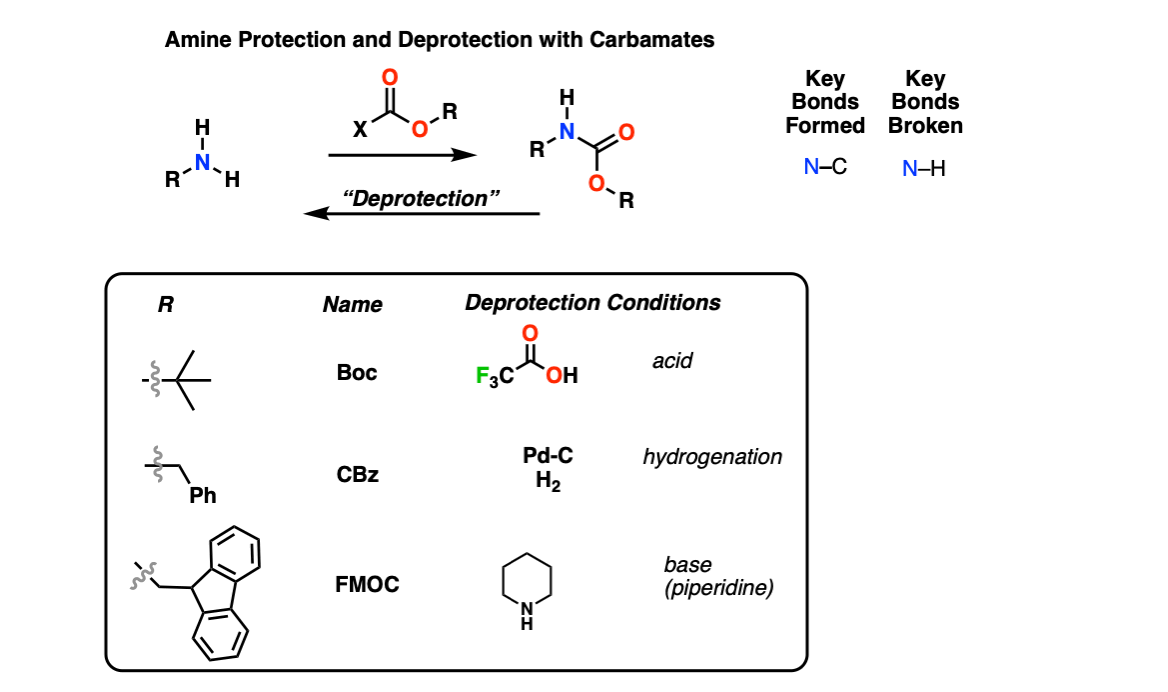
Amine Protection and Deprotection Master Organic Chemistry
A protecting group or protective group is introduced into a molecule by chemical. Deprotection is the process of removing a protective group from a molecule, typically an. Protecting groups are needed to temporarily block a certain reactive site on a molecule. Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. When the alcohol is protected with.
Amine Protection and Deprotection Master Organic Chemistry
Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. Protecting groups are needed to temporarily block a certain reactive site on a molecule. Deprotection is the process of removing a protective group from a molecule, typically an. When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones. A protecting.
organic chemistry Mercury assisted deprotection of dithiane
Protecting groups are needed to temporarily block a certain reactive site on a molecule. When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones. Stability data for the most frequently used protective groups, protection and deprotection. A protecting group or protective group is introduced into a molecule by chemical. Deprotection is the process.
Amine Protection and Deprotection Master Organic Chemistry
A protecting group or protective group is introduced into a molecule by chemical. Deprotection is the process of removing a protective group from a molecule, typically an. Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. Protecting groups are needed to temporarily block a certain reactive site on a molecule. When the alcohol is protected with.
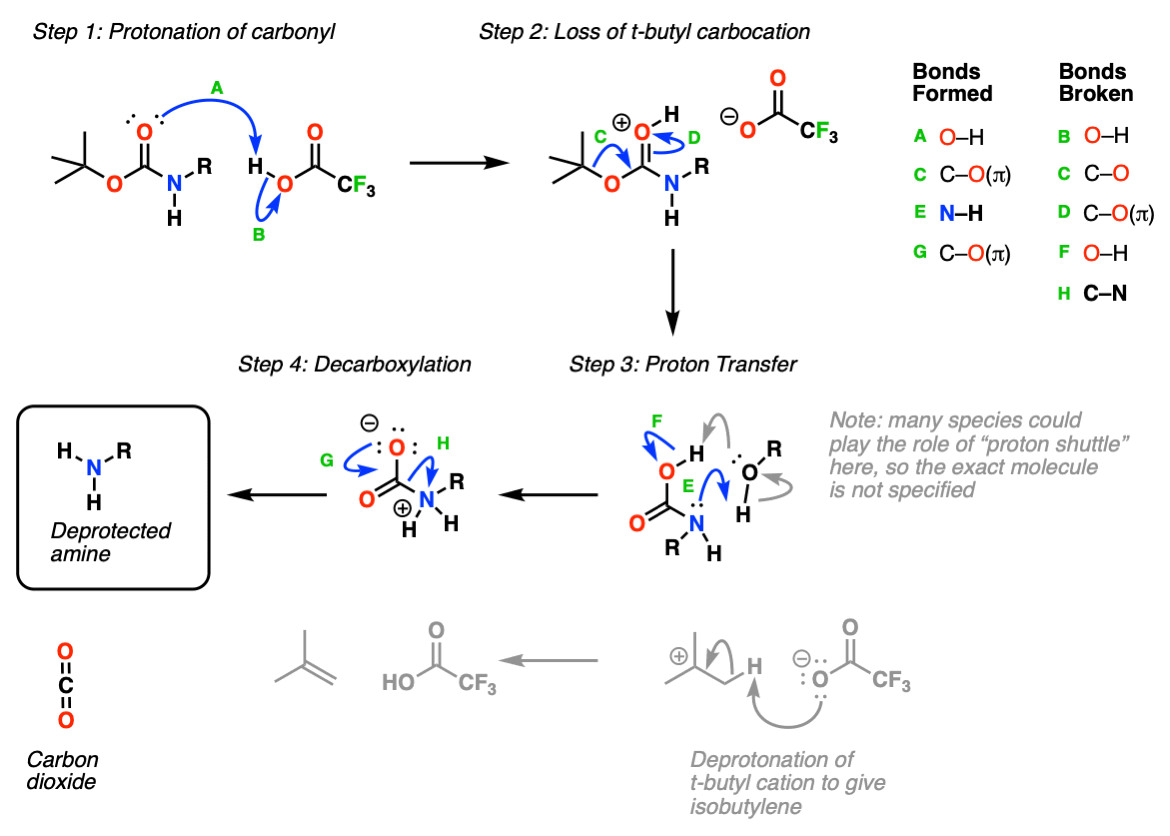
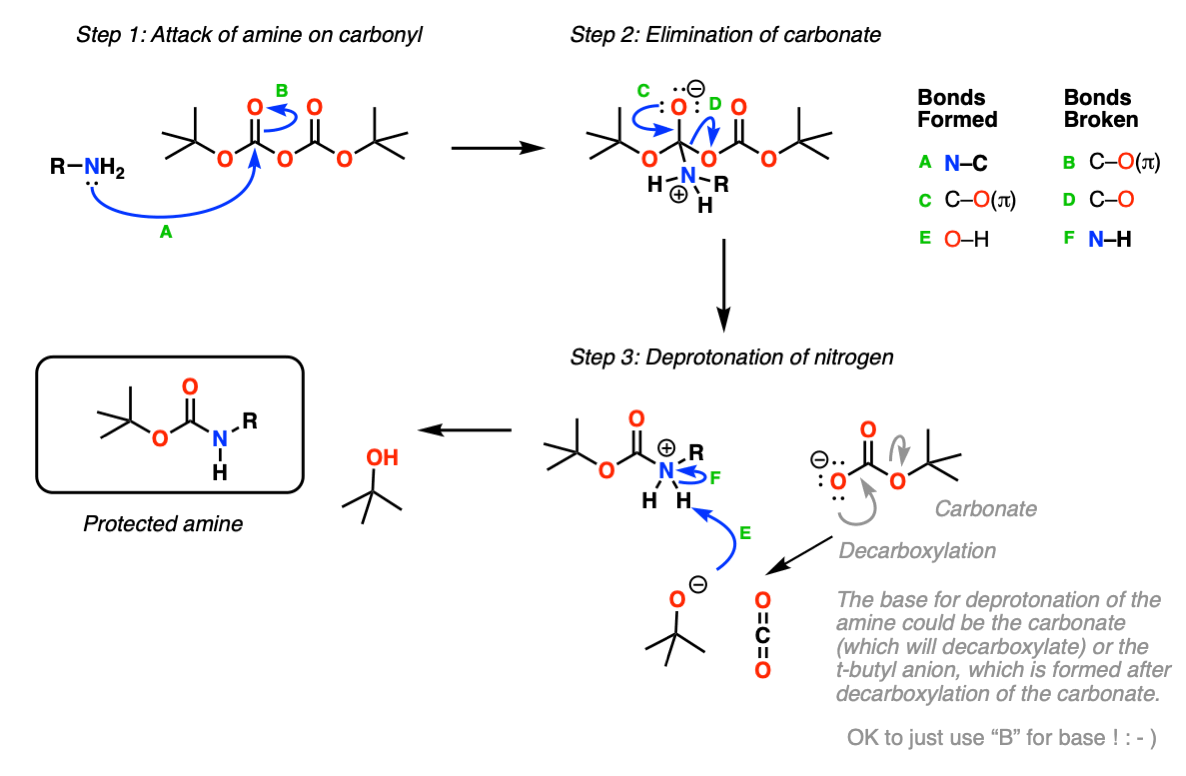
Boc Protecting Group for Amines Chemistry Steps
Protecting groups are needed to temporarily block a certain reactive site on a molecule. When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones. A protecting group or protective group is introduced into a molecule by chemical. Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. Stability data for.
Protecting Groups For Alcohols Master Organic Chemistry
Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. Protecting groups are needed to temporarily block a certain reactive site on a molecule. Deprotection is the process of removing a protective group from a molecule, typically an. When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones. Stability data.
Regioselective Anomeric O‐Benzyl Deprotection in Carbohydrates
A protecting group or protective group is introduced into a molecule by chemical. Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. Stability data for the most frequently used protective groups, protection and deprotection. When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones. Protecting groups are needed to.
Amine Protection and Deprotection Master Organic Chemistry
Stability data for the most frequently used protective groups, protection and deprotection. Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. A protecting group or protective group is introduced into a molecule by chemical. When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones. Protecting groups are needed to.
Stability Data For The Most Frequently Used Protective Groups, Protection And Deprotection.
Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one. Deprotection is the process of removing a protective group from a molecule, typically an. When the alcohol is protected with a t bdms group, only the aldehyde is oxidized by jones. Protecting groups are needed to temporarily block a certain reactive site on a molecule.