Which Of The Following Forms A Molecular Solid - Which of the following is considered a molecular solid? A molecular solid is a solid composed of atoms or molecules bound. Hydrogen atom of one water molecule forms hydrogen bonds with oxygen atom of. In the given options, calcium fluoride and sodium chloride are the ionic crystals due to presence. Which of the following forms a molecular solid? To determine which of the given substances forms a molecular solid, identify the type of. Which of the following is considered an. Molecular solids are formed from molecules that are held together by weaker. Study with quizlet and memorize flashcards.
To determine which of the given substances forms a molecular solid, identify the type of. In the given options, calcium fluoride and sodium chloride are the ionic crystals due to presence. Hydrogen atom of one water molecule forms hydrogen bonds with oxygen atom of. Which of the following forms a molecular solid? Molecular solids are formed from molecules that are held together by weaker. Which of the following is considered a molecular solid? A molecular solid is a solid composed of atoms or molecules bound. Which of the following is considered an. Study with quizlet and memorize flashcards.
A molecular solid is a solid composed of atoms or molecules bound. To determine which of the given substances forms a molecular solid, identify the type of. In the given options, calcium fluoride and sodium chloride are the ionic crystals due to presence. Which of the following forms a molecular solid? Study with quizlet and memorize flashcards. Hydrogen atom of one water molecule forms hydrogen bonds with oxygen atom of. Which of the following is considered an. Molecular solids are formed from molecules that are held together by weaker. Which of the following is considered a molecular solid?
Solved Which of the following is probably a molecular solid?
Hydrogen atom of one water molecule forms hydrogen bonds with oxygen atom of. A molecular solid is a solid composed of atoms or molecules bound. In the given options, calcium fluoride and sodium chloride are the ionic crystals due to presence. Molecular solids are formed from molecules that are held together by weaker. Which of the following forms a molecular.
Matter exists in three physical forms solid liquid Tutorix
Which of the following is considered an. In the given options, calcium fluoride and sodium chloride are the ionic crystals due to presence. Hydrogen atom of one water molecule forms hydrogen bonds with oxygen atom of. Molecular solids are formed from molecules that are held together by weaker. To determine which of the given substances forms a molecular solid, identify.
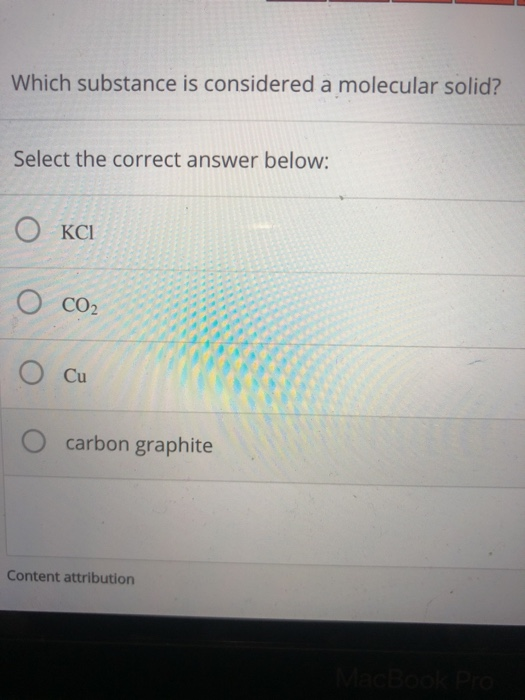
Solved Which substance is considered a molecular solid?
Study with quizlet and memorize flashcards. Which of the following is considered a molecular solid? Which of the following forms a molecular solid? To determine which of the given substances forms a molecular solid, identify the type of. Hydrogen atom of one water molecule forms hydrogen bonds with oxygen atom of.
Molecular solidstate structure of one of the two independent
To determine which of the given substances forms a molecular solid, identify the type of. A molecular solid is a solid composed of atoms or molecules bound. Which of the following is considered an. Hydrogen atom of one water molecule forms hydrogen bonds with oxygen atom of. Molecular solids are formed from molecules that are held together by weaker.
Molecular solids formation, properties, crystal structure and uses
Which of the following is considered a molecular solid? Which of the following is considered an. Hydrogen atom of one water molecule forms hydrogen bonds with oxygen atom of. Molecular solids are formed from molecules that are held together by weaker. In the given options, calcium fluoride and sodium chloride are the ionic crystals due to presence.
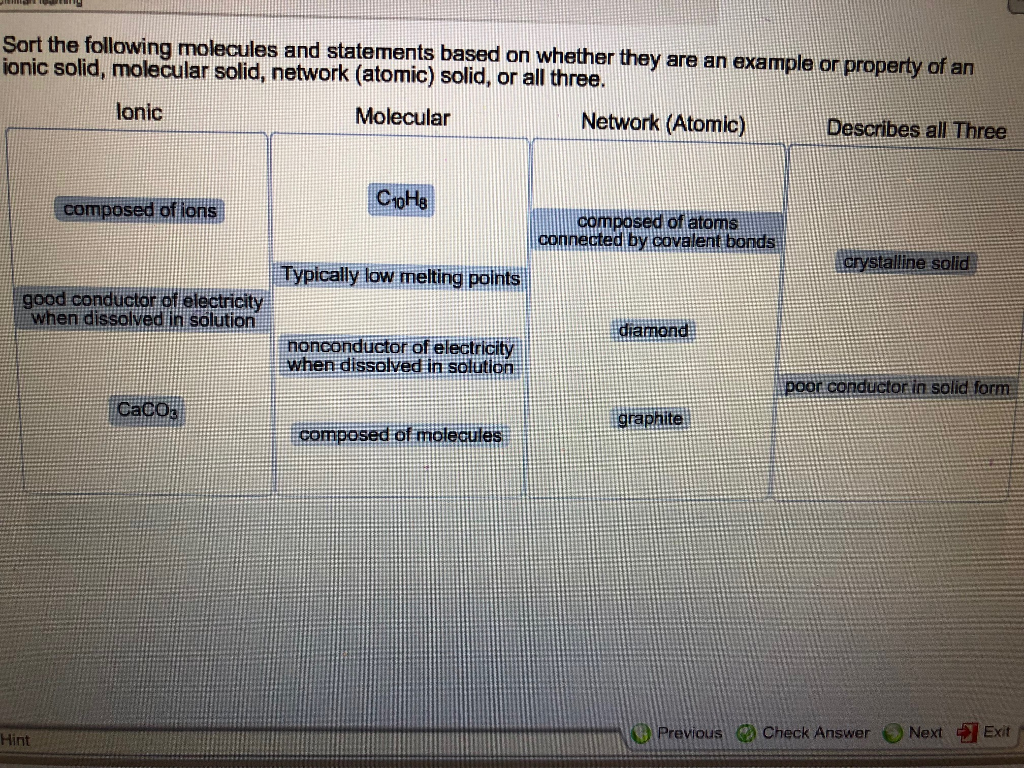
Solved Sort the following molecules and statements based on
In the given options, calcium fluoride and sodium chloride are the ionic crystals due to presence. Which of the following forms a molecular solid? Molecular solids are formed from molecules that are held together by weaker. To determine which of the given substances forms a molecular solid, identify the type of. Which of the following is considered a molecular solid?
4. Molecular Shapes
Hydrogen atom of one water molecule forms hydrogen bonds with oxygen atom of. Which of the following is considered an. Study with quizlet and memorize flashcards. Which of the following is considered a molecular solid? In the given options, calcium fluoride and sodium chloride are the ionic crystals due to presence.
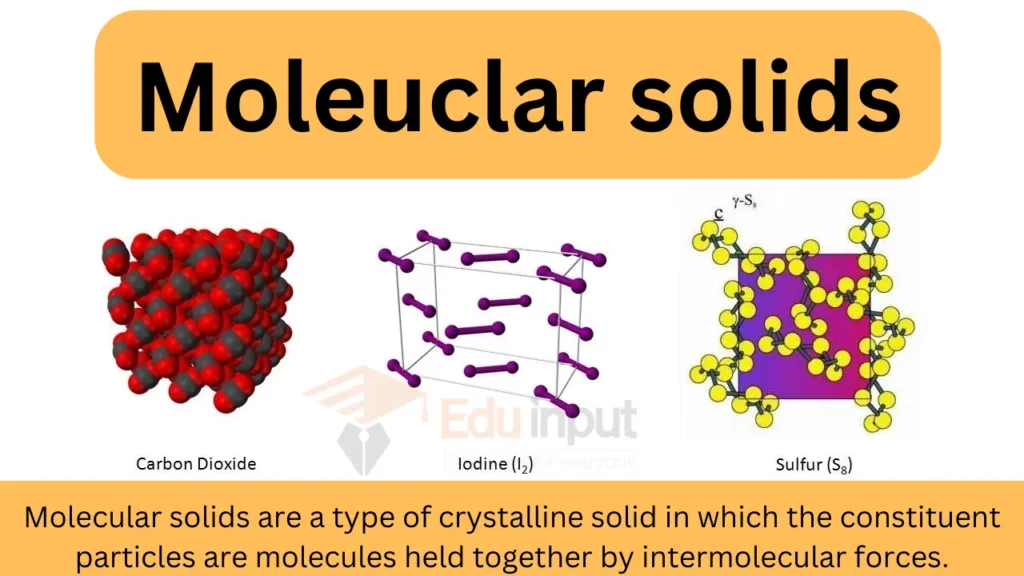
Molecular Solid Examples
Which of the following is considered a molecular solid? To determine which of the given substances forms a molecular solid, identify the type of. Study with quizlet and memorize flashcards. Which of the following forms a molecular solid? Which of the following is considered an.
Molecular Solid Examples
Which of the following is considered an. To determine which of the given substances forms a molecular solid, identify the type of. Which of the following is considered a molecular solid? In the given options, calcium fluoride and sodium chloride are the ionic crystals due to presence. A molecular solid is a solid composed of atoms or molecules bound.
Molecular Solid Definition and Examples
A molecular solid is a solid composed of atoms or molecules bound. Molecular solids are formed from molecules that are held together by weaker. Which of the following is considered an. Which of the following is considered a molecular solid? To determine which of the given substances forms a molecular solid, identify the type of.
Hydrogen Atom Of One Water Molecule Forms Hydrogen Bonds With Oxygen Atom Of.
Study with quizlet and memorize flashcards. A molecular solid is a solid composed of atoms or molecules bound. To determine which of the given substances forms a molecular solid, identify the type of. In the given options, calcium fluoride and sodium chloride are the ionic crystals due to presence.
Which Of The Following Forms A Molecular Solid?
Which of the following is considered an. Which of the following is considered a molecular solid? Molecular solids are formed from molecules that are held together by weaker.




/a-spoonful-of-sugar-154394355-58a8e93e5f9b58a3c945690d.jpg)